Sulphur!!- the VULCANIZING AGENT
December 01, 2022 · ASSOCIATED RUBBER CHEMICALS (KOCHI) PVT LTD
VULCANIZING AGENTS
Sulphur is a multivalent non-metal, abundant, tasteless and odourless. In its native form sulphur is a yellow crystalline solid. In nature it occurs as the pure element or as sulfide and sulfate minerals.
Elemental sulphur is used in black gunpowder, matches, and fireworks; in the vulcanization of rubber; as a fungicide, insecticide, and fumigant; in the manufacture of phosphate fertilizers; and in the treatment of certain skin diseases.
Sulphur vulcanization is a chemical process for converting natural rubber or related polymers into materials of a variety of hardness, elasticity, and mechanical durability by heating them with sulphur or other equivalent curatives or accelerators.
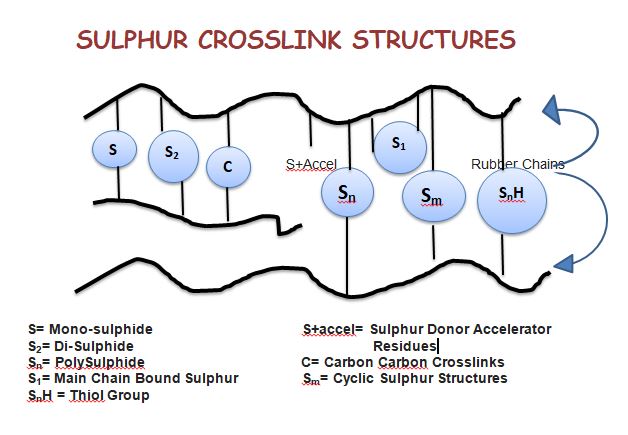
The sulphur vulcanization was discovered in 1839 by Charles Goodyear. During vulcanization, the rubber molecule becomes cross-linked and a three-dimensional network structure is formed. During vulcanization rubber loses its tackiness and it is more resistant to solvents, heats, light, etc. Fig shows the schematic representation of sulphur vulcanization. Check out Moulded Rubber products manufacturers in Kerala
Vulcanization involves chemical procedure wherein rubber is blended with activators, accelerator, and Sulfur at the temperature of 140–160 °C. Cross-linking occurs among long molecules of rubber, to add up the tensile strength, tenderness, and resilience toward weather.
Quick Enquiry
To know more about Associated Chemicals feel free to send a message
 Our Sister Concerns
Our Sister Concerns 


Usefull Links
Get In Touch
Assochem Chambers, Bypass, Edapally,
Kochi-682024, Kerala, India.
Phones : +91 9495999349, +91 9388610189, +91 484 2339190, +91 484 2348028
E-mail : nsn@assochem.in, marketing@assochem.in, mail@assochem.in
Support