
Rubber Vulcanization
October 05, 2022 · Associated Rubber Chemicals (Kochi) Pvt Ltd
The vulcanization process of the rubber was discovered in 1839 and the individuals responsible for this discovery were Charles Goodyear in USA and Thomas Hancock in England. Both discovered the use of Sulfur and White Lead as a vulcanization system for Natural Rubber. This discovery was a major technological breakthrough for the advancement of the world economy. Here, we can have a look at the vulcanization process, the chemicals used for vulcanizing and the accelerators.
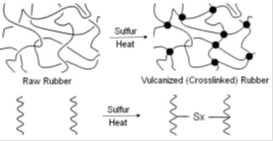
Vulcanization is a cross linking process in which individual molecules of rubber (polymer) are converted into a three dimensional network of interconnected (polymer) chains through chemical cross links(of sulfur). Vulcanization of rubbers by sulfur alone is an extremely slow and inefficient process. The chemical reaction between sulfur and the Rubber Hydrocarbon occurs mainly at the C = C (double bonds) and each crosslink requires 40 to 55 sulphur atoms (in the absence of accelerator). The process takes around 6 hours at 140°C for completion, which is uneconomical by any production standards. The vulcanizates thus produced are extremely prone to oxidative degradation and do not possess adequate mechanical properties for practical rubber applications. These limitations were overcome through inventions of accelerators which subsequently became a part of rubber compounding formulations as well as subjects of further R&D. Following is the summary of events which led to the progress of ‘Accelerated Sulfur Vulcanization'.In this paper let us discuss about the chemicals used for the vulcanizing and its accelerators. Check out the best Moulded rubber products Manufacturer in Kerala.
Sulfur remains the most successful and economical cross linking agent even today! Sulfur as vulcanizing agent has a limitation that, the elastomers must contain chemical unsaturation (C=C double bonds) for sulfur cross linking. The structure of speciality elastomers EPDM and Butyl had to be chemically modified to make sulfur vulcanization possible for their commercial success. Other chemicals used for cross linking of polymers are Sulfur Monochloride, Tellurium, Selenium, Thiuram accelerators, Polysulphide polymers, p-Quinonedioximes, Metallic Oxides, Organic Peroxides, Di-isocyanates, etc. (mostly for specialized applications). In the case of fully saturated elastomers organic peroxides are often used for cross linking.
Accelerators
An accelerator is defined as the chemical added into a rubber compound to increase the speed of vulcanization and to permit vulcanization to proceed at lower temperature and with greater efficiency.
Accelerator also Decreases the Quantity of Sulphur necessary for vulcanization and thus improving 'aged' properties of the rubber vulcanizates.
Accelerators are also classified as Primary and / or Secondary accelerators based on the role they play in a given compound.
Generally, Thiazoles and Sulfenamide accelerators play a role of being Primary Accelerators due to their characteristics such as good processing safety, a broad vulcanization plateau and optimum cross link density as well as desired reversion delay that they offer. The Primary Accelerators are used at 0.5 to 1.5 phr dosages in most rubber compounds.
The basic accelerators such as Guanidines, Thiurams, and Dithiocarbamates etc are used as Secondary accelerators to activate the primary accelerators. The use of secondary accelerators increases the speed of vulcanization substantially but at the expense of scorch safety. The dosages of the secondary accelerators are generally between 10-40% of the primary accelerator
| Accelerators | Chemical Group | Vulcanization Speed |
| BA, HMT | Aldehyde Amine | Slow |
| DPG, DOTG | Guanidine | Slow |
| MBT, MBTS, ZMBT | Thiazole | Semi Ultra fast |
| ZBDP | Thiophosphate | Ultra fast |
| CBS, TBBS, MBS, DCBS | Sulfenamides | Fast-Delayed action |
| ETU, DPTU, DBTU | Thiourea | Ultra fast |
| TMTM, TMTD, DPTT, TBzTD | Thiuram | Ultra fast |
| ZDMC, ZDEC, ZDBC, ZBEC | Dithiocarbamate | Ultra fast |
| ZIX | Xanthates | Ultra fast |
Selection of Accelerators for Rubber Compounds:
Before selecting an Accelerator system for the manufacture of a particular rubber product, following points have to be taken into account.
- Solubility in rubber (high solubility required to avoid bloom & improve dispersibility),
- Processing operations & their temperatures the rubber compound is be required to undergo,
- Adequate Scorch Time desired for 'scorch free' processing & storage stability,
- The Cure rate requirements,
- Reversion characteristics desired (delayed reversion on over cure),
- Vulcanization method to be used (mode of heat transfer),
- Maximum vulcanization temperature available,
- Cure cycle desired at the available vulcanisation method and temperature,
- Requirements of vulcanizates properties (to decide the type & state of cure),
- Effectiveness over a wide range of cure temperatures & Suitability for use with different polymer blends,
- No adverse effects on other properties / materials (e.g. bonding, ageing, adhesion, non-rubber components in the rubber product),
- No known health hazards upon usage as chemical / its decomposition products and easy to handle nd dust suppressed physical form.
- No adverse effects during end-use of the rubber product (e.g. accelerators used in the manufacture of rubber articles intended for food contact / surgical use),
- Stability of the accelerator as a chemical.
Vulcanization System (Cure system):
A typical sulfur vulcanization system consists of following ingredients:
Zinc oxide 3.0 to 10 phr
Stearic acid 1.0 to 4 phr
Accelerator 0.5 to 4 phr
Sulfur 0.5 to 3 phr
In this vulcanization system; for a particular rubber compound, selection of appropriate accelerators is a very important task since both the accelerator type as well as dosage greatly influence processing and vulcanizate properties of the rubber compound.
Quick Enquiry
To know more about Associated Chemicals feel free to send a message
 Our Sister Concerns
Our Sister Concerns 


Usefull Links
Get In Touch
Assochem Chambers, Bypass, Edapally,
Kochi-682024, Kerala, India.
Phones : +91 9495999349, +91 9388610189, +91 484 2339190, +91 484 2348028
E-mail : nsn@assochem.in, marketing@assochem.in, mail@assochem.in
Support

















