
Prevulcanised Latex
October 17, 2022 · Preethy Paul
Introduction
Natural Rubber Latex (NRL) obtained from the rubber tree Hevea brasiliensis is a colloidal suspension of rubber particles in an aqueous serum. The aqueous medium contains a number of dissolved non rubber substances like amino acids, proteins, carbohydrates, organic acids, inorganic salts etc.
Field latex coagulates 6 to 10 hours after tapping. Anti coagulants are normally added to latex depending on the final product to which field latex is processed into.
For processing into 60% centrifuged latex, ammonia, ZnO, TMTD etc are used as preservatives. The field latex is then centrifuged into 60% concentrate (CL 60). This CL 60 forms the raw material for making prevulcanised latex.
CL 60 has the following basic spec
drc 60%
NH3 0.07% (High Ammonia)
0.03% (Low Ammonia)
There are a number of other properties which will be elaborated in the course of the paper.
Check out Moulded rubber product manufacturers in Kerala
Prevulcanised Latex
Prevulcanised Latex is defined as one in which the rubber particles are chemically cross linked and remains in fluid state so that on drying the latex a vulcanized film is obtained. The possibility of vulcanizing the disperse phase of NRL without any prior coalesce of particles was first investigated by Schidrowitz in 1914 – 1918 period.
His method of prevulcanisation was to heat NRL with sodium poly sulphide, sulphur and zinc oxide in steam at 145oC for 30 to 45 minutes. At the conclusion of the process the latex was cooled, sedimented, sieved and packed. Later, when high speed water soluble accelerators where available the curing condition was altered to 1 hour at 70-80oC preceded by a 1 hour rise to that temperature.
Methods of Prevulcanisation
The three common methods of prevulcanisation are
- Sulphur vulcanization
- Peroxide vulcanization
- High energy radiation vulcanization
Since the economical and most common method of prevulcanisation is the sulphur vulcanization, the discussio9n will be confined to this method.
Current methods of sulphur prevulcanisation
On a broad classification, the three methods of sulphur prevulcanisation are
1. Cold Fast Process
Latex is stabilized, excess vulcanizing agents area added, maintained at room temperature for 6 to 8 days, excess chemicals removed.
2. Hot Fast Process
Latex is stabilized, excess vulcanizing agents added, heated to 70 to 80oC, for 2 to 4 hours, cooled, excess chemicals removed.
3. Cold Slow Process
Latex is stabilized, required chemicals are added, maintained at room temperature for 30 to 45 days, practically no unreacted chemicals.
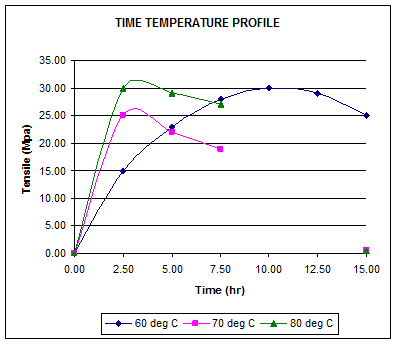
Time – Temperature Profile
The Time – Temperature profile of sulphur prevulcanisation can be represented as shown
Monitoring of Prevulcanisation level
The three common tests to monitor the prevulcanisation levels are
- Chloroform test
- Toluene swell test
- Determination of modulus
The realistic quantitative measurement of cure is undoubtedly determination of modulus at 100% elongation. The modulus increases progressively as the cure proceeds. The drawback of this procedure is the time taken for the test.
Chloroform test
This is a very simple test and a rough method for ascertaining the extent of cure. A small volume of latex is mixed with an equal volume of chloroform until coagulation occurs. The physical state of the coagulum gives an indication of the extent of cure.
Chloroform No. 1. Unvulcanised latex gives a coagulum which is soft, extensible and coherent.
Chloroform No. 2. The coagulum is a weak lump which breaks short when stretched
Chloroform No. 3. The coagulum has the form of nontacky agglomerates
Chloroform No. 4. Completely vulcanized latex gives a dry, crumbly coagulum
The higher the state of cure the less will be the tendency of the rubber particle to coalesce and fuse together when swollen with choloform.
Toluene swell test
This is a more satisfactory and fairly rapid method of determining the cure of a compound. The procedure consists of first making a dried down film 0.003 to 0.01 mm thick from the prevulcanised latex. A 1” dia disc is then cut out from it and immersed in a typical swelling agent like Toluene taken in a Petri dish. After 25 to 27 minutes of immersion the swelled dia is measured. This is done by placing the disc on a graph paper.
Toluene Swell TS = (Ds – Do) X 100
Do
Toluene swell Index TSI = (Ds)2 - (Do)2
(Do)2
Where Do = diameter original
Ds = diameter swelled
Modulus Test
The modulus test is the most accurate method of determining the level of cure of a compound. This test consists of casting a thin film of latex and a test piece is cut from it. The modulus is usually determined at 100% extension, 500% extension and 700% extension.
Mechanism of Prevulcanisation
Many theories have been put forward on the mechanism of prevulcanisation, but little is known about the actual method.
In appearance the prevulcanised latex is similar to unvulcanised latex, retaining its original fluidity. It is assumed that vulcanisation takes place in individual latex particle without altering their state of dispersion. The particles retain its shape and size. It is an intra particle crosslinking of the elastomer within the disperse phase that takes place while still retaining the integrity and stability of the colloidal system over long periods of time.
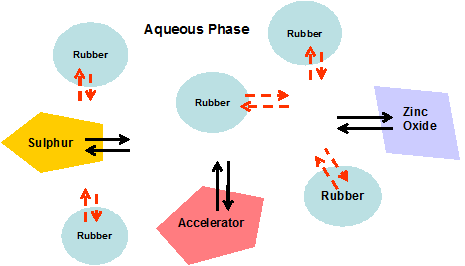
Schematic illustration of sulphur-prevulcanization reaction system
Prof. D.C. Blackley – Polymer Latices Vol. 3
It is assumed that the vulcanizing ingredients are absorbed into the rubber particles from the aqueous phase and not by direct contact between the rubber particles of rubber and particles of vulcanizing ingredients. These ingredients may be imagined as portioned between the aqueous phase and rubber phase.
For sulphur prevulcanisation to take place at extremely low temperatures the presence of water is necessary. The reaction is believed to be accelerated by small amounts of additional substances in particular the non rubber constituents present in normal ammonia preserved natural rubber latex concentrate.
It appears that the first step of the reaction is the formation of a sulphur-accelerator species in the aqueous phase of latex. This species or a derivative of it then transfers to the rubber phase; possibly adsorption to the rubber phase from the water phase of latex. The sulphur-accelerator species has to be soluble in the aqueous medium. It could be rendered soluble in water to a sufficient extent by complexation with some of the water soluble non rubber constituents present in normal ammonia preserved natural latex concentrate. The rate of prevulcanisation is reduced if most of the non rubber solids are removed from natural rubber latex.
The mechanism by which the sulphur-accelerator species enters the interior of the rubber particle is most possibly by sulphur-accelerator losing by dissociation some or all of the molecules that have rendered it sufficiently water soluble for transfer to the surface of the rubber particle. The lost molecules return to the aqueous phase. The residual sulphur-accelerator species at the particle surface would be sufficiently hydrophobic to migrate to the interior of the rubber particle.
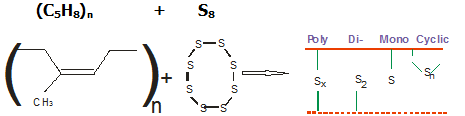
Further a sequence of reactions occur which results in the rubber molecules becoming crosslinked by polysulphidic bridges.
The various sulphur linkages can be illustrated as below
The proposed mechanism by C C Ho and M C Knew is:
The compounding ingredients are absorbed to the latex particles in colloidal suspension. The vulcanisation initiates at the outermost surface of the latex particles and gradually propagates inner. The outer layer often crosslinks more than the inner layer.
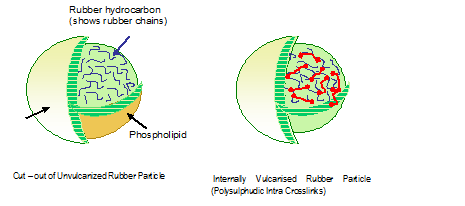
The above figure shows 2 droplets at the early stage of prevulcanisation. The figure below illustrates how the latex droplets coalesce on drying and inter droplet crosslinks develop.
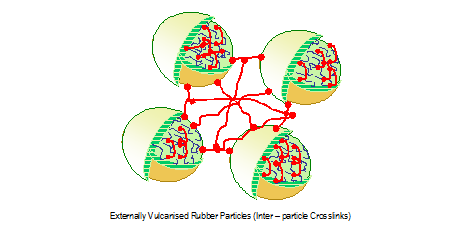
Structure of films derived from sulphur prevulcanised natural rubber latex
Three different theories have been proposed to explain the strength and coherence of films derived from prevulcanised latex.
- The primary valance bond theory
- The bonding adhesive theory
- The secondary valance bond theory
1. The primary valance bond theory
This theory proposes the formation of inter particle covalent chemical bonds between macromolecules of rubber as the film dries
2. The bonding adhesive theory
In this theory the strength is attributed to the closed packed assemblage of small particles of unvulcanised rubber bonded by non rubber substances situated in the interface between the particles. This theory has no credence because in practice we find that the films of sulphur prevulcanised latex attains strength on better leaching when the non rubber constituents are removed.
3. The secondary valance bond theory
According to this theory the bonding is by way of secondary valence attractive interactions between segments of rubber macro molecules. This is depicted below
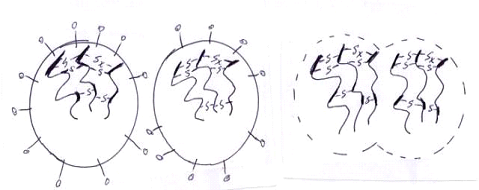
At final stage of prevulcanisation Coalesce and crosslink
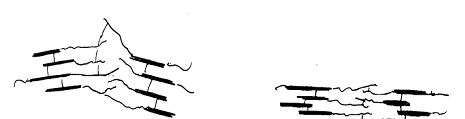
Film after coalesce and crosslink Orientation during stretch
Particle Size Analysis of CL 60 and MR C (Prevulcanised Latex)
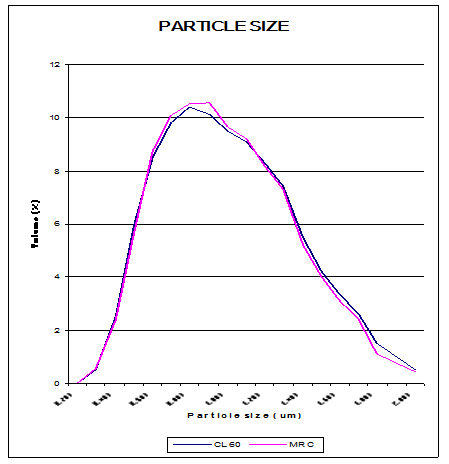
This graph depicts the particle size of rubber molecules in CL 60 and MR C grades. It can be seen that the particle size distribution of both the grades are almost the same thus giving credence to the theory that during prevulcanisation it is the intra molecular crosslinks that are formed and not the inter molecular cross links. If the inter molecular crosslinks are formed the size of the rubber particles would have increased.
Good Practice in NRL Compounding
- Particle size of chemical dispersion must be comparable to particle size of latex
- They must be capable of mixing intimately
- Particle size of compounding ingredients must be of dia 1 – 2 μm
- Lower chemical particle sizes are important for dipped fine film products
- Colloidal stability of dispersions must match that of latex. Dissolved substances can adversely affect the colloidal stability of latex.
- pH of dispersions and latex must be same to prevent disturbance to equilibrium.
- Ionic strength of the aqueous phase of dispersion should be similar to the latex. The electrolytes that give rise to ionic strength must be in equilibrium.
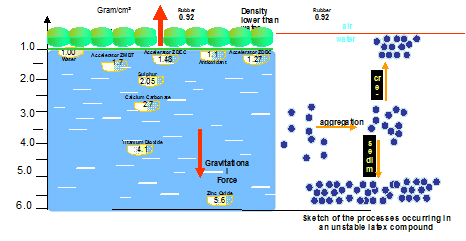
- The varying densities of rubber chemicals and the very low density of rubber is a serious restriction in compounding leading to aggregation and sedimentation.
This is depicted in the diagram below.
Process advantage in using Prevulcanised Latex
Today Prevulcanised Latex is readily available in the market and it has distinct advantages over in-house compounding.
- Ready to use hence only just in time inventory required
- Usually processed in large lots hence lesser batch to batch variations
- No inventory of chemicals need be maintained
- Risk of batch failures not there
- Problem of Zinc Amine thickening not there
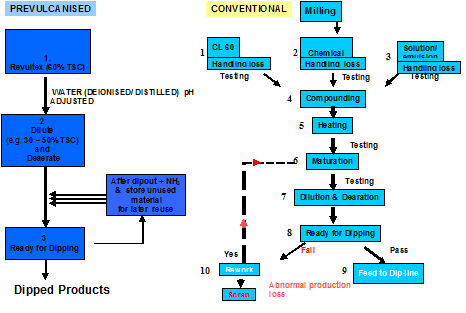
- Ease of operation
The ease of operation is detailed below
Grades of Prevulcanised Latex
Commercially Prevulcanised Latex is available in three primary grades and many other derivatives. The primary grades are;
LM - Low modulus
MM - Medium Modulus
HM - High Modulus
The typical properties of these grades are
| Grade | Tol Swell | Tensile | Modulus 700% | EB |
| LM | 90 - 109 | 28 | 9 | 950 |
| MM | 85 - 89 | 25 | 13 | 850 |
| HM | 82 - 84 | 27 | 17 | 815 |
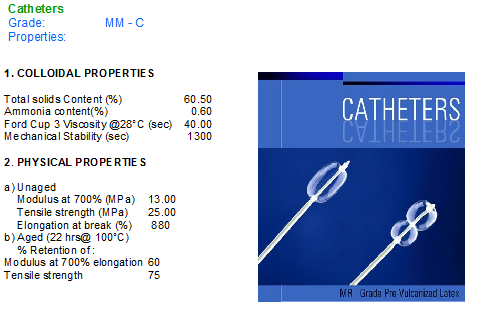
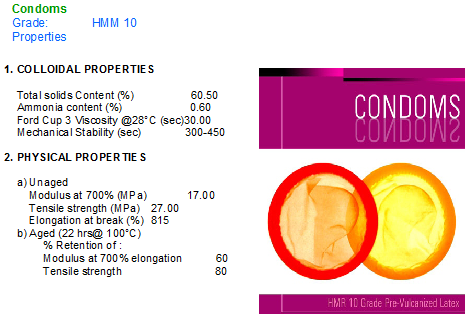
We “Associated” offers ready to use/PV latex for the following Products


Quick Enquiry
To know more about Associated Chemicals feel free to send a message
 Our Sister Concerns
Our Sister Concerns 


Usefull Links
Get In Touch
Assochem Chambers, Bypass, Edapally,
Kochi-682024, Kerala, India.
Phones : +91 9495999349, +91 9388610189, +91 484 2339190, +91 484 2348028
E-mail : nsn@assochem.in, marketing@assochem.in, mail@assochem.in
Support

















